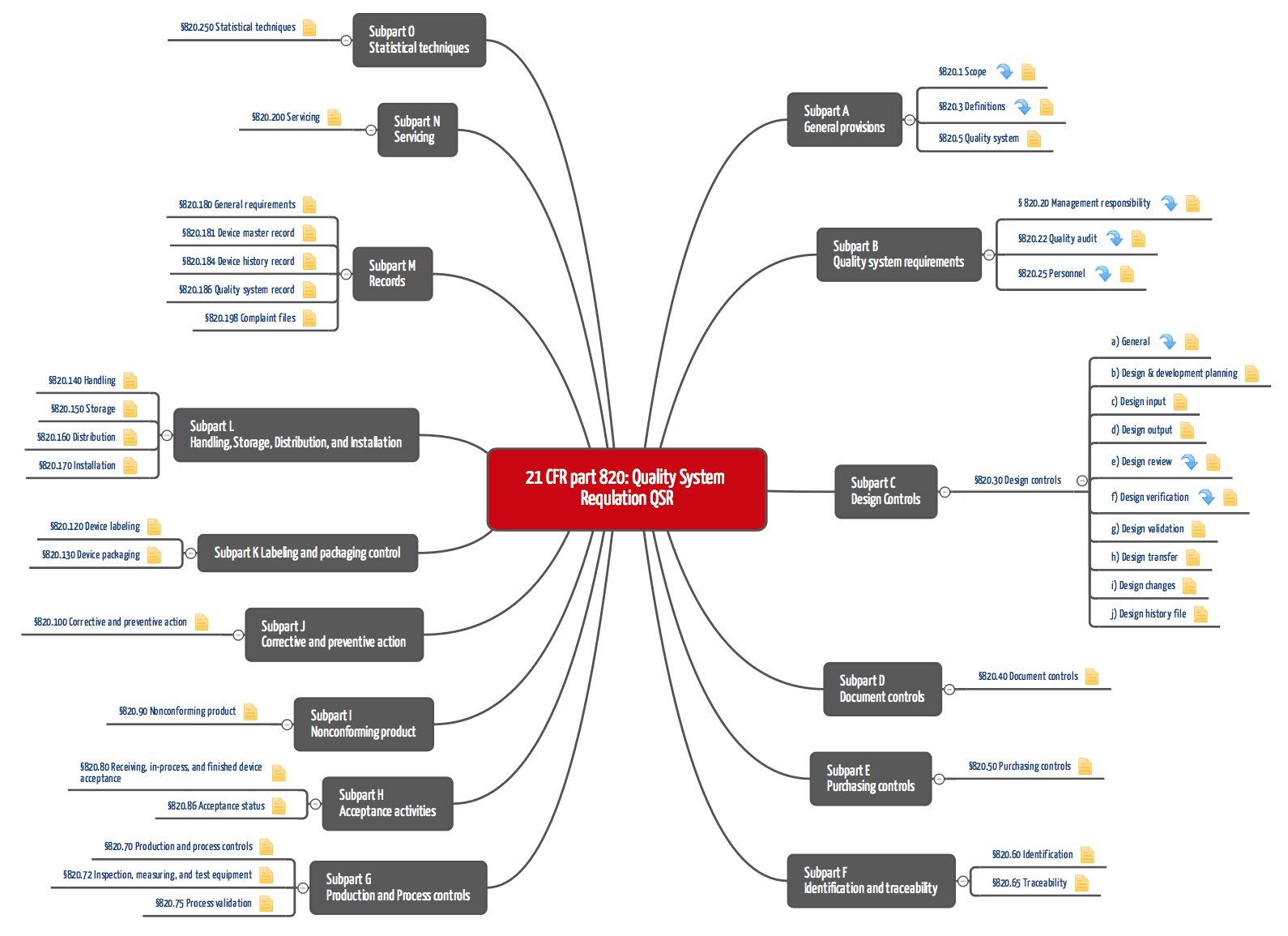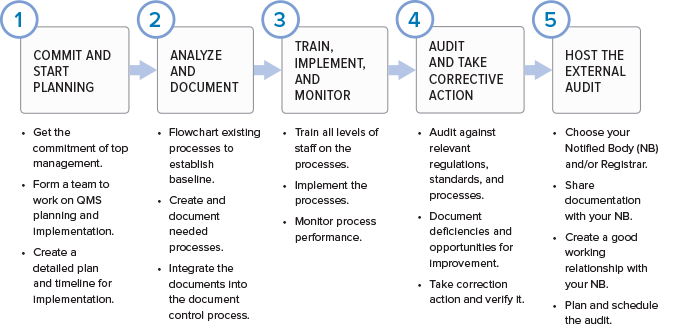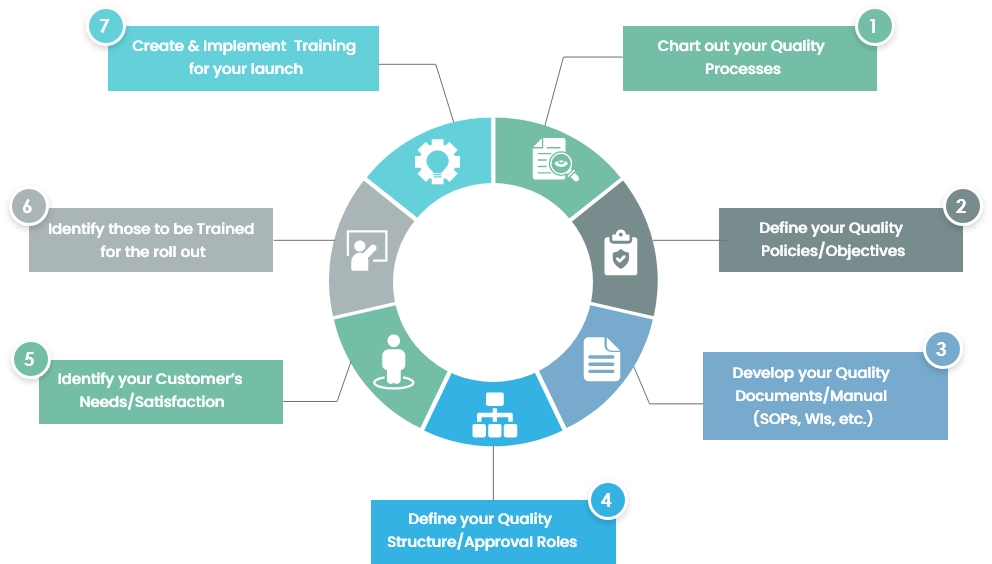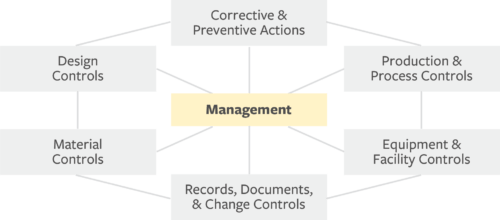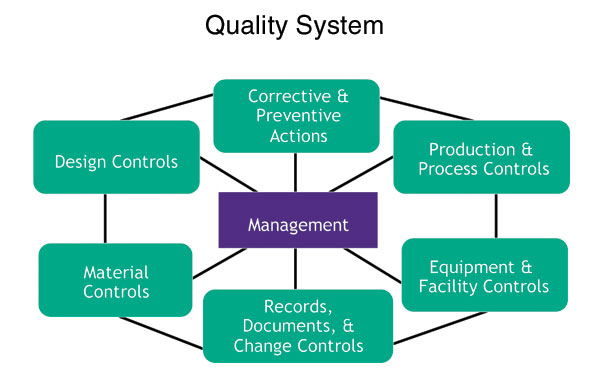
Medical device makers find solution to FDA demands - Tooling and Production Magazine for Large Plant Management and Metalworking

End-to-End Biologics CDMO Quality Management System|OPM Biosciences – Cell Culture Media and End-to-End CDMO

Quality Control for Pharmaceutical Packaging Production - Pharmaceutical Quality Management Systems | Aphena Pharma Solutions

Designing A World-Class Quality Management System For FDA Regulated Industries: Quality System Requirements (QSR) For cGMP : Muchemu, David: Amazon.fr: Livres
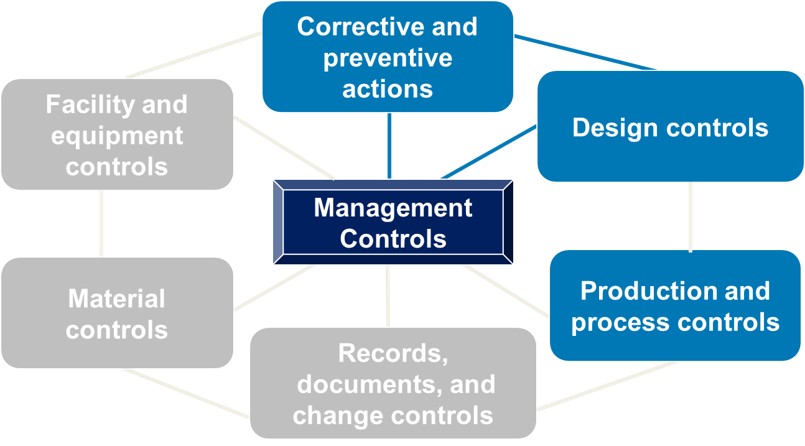

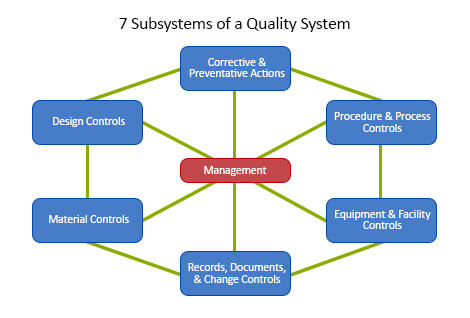

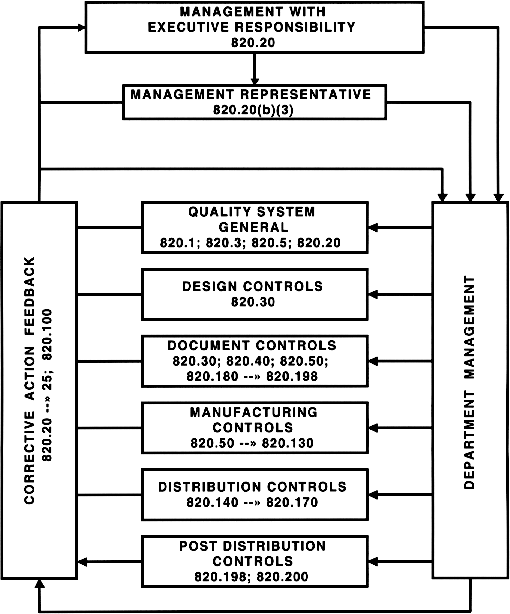
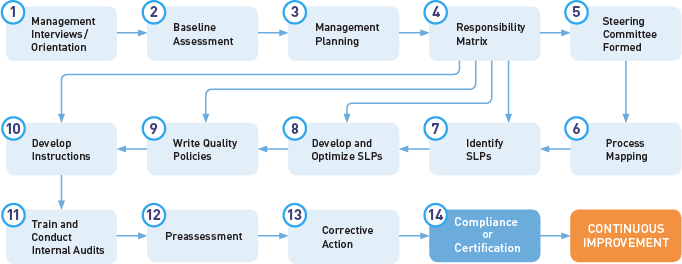

.webp?width=500&height=381&name=Quality%20management%20system%20product%20development%20(1).webp)